If you're pressed for time and don't want to read the entire post, just read the abstract.
Abstract: When God designed the atom, He had worked a few too many late nights.
Let's go down to the fundamental basics. The basic units of matter are known as elementary particles, such as quarks (which combine in certain combinations to create protons and neutrons) and leptons (such as electrons). We'll take a look at both of these particles.
Quarks come in six flavors: up, down, strange, charm, top and bottom. Each one has a slight distinction that makes it different from the rest, but we'll just focus on the ones that can be found within the nucleus--the up and down quarks. Here's a little table that lists the actual characteristics of each flavor, and then I'll explain what each term means:
| Mass | Spin | Charge | |
| Up | 1.5-3.3 MeV | 1/2 | +2/3 |
| Down | 3.5-6.0 MeV | 1/2 | -1/3 |
(Source: ParticleZoo for iPad)
Although the mass category may seem to be somewhat self-explanatory, it's important to notice that the mass here is measured in mega electron volts. An electron volt is simply a unit of energy (in the quantum world, mass and energy are equivalent, after all--that's why it's called "mass-energy equivalence").
"Spin," however, takes a bit more dedication to wrap our minds around. The "spin" of a quark--or, for that matter, any particle--is a fundamental factor of a particle's quantum state. It was originally thought to be an actual rotation around an axis. This is only correct in that the spin of a particle follows the same mathematical rules as rotation. My understanding of it is that it is a mathematical convenience--a property that needs to be described, but the actual details are not yet clear.
"Charge" is somewhat more intuitive. It simply refers to the attraction or repulsion between different particles. (Although the fact that quarks have fractional charge values is not what we would expect, this can be explained in part by the fact that quarks have never been found without being coupled with other quarks that provide a discrete, integer value.)
The varying charges of up and down quarks provide us with a way of explaining the charges of a proton and a neutron. When two up quarks and one down quark combine (via the strong interaction), the total charge is simply:
$\frac{2}{3}+\frac{2}{3}-\frac{1}{3}=1$
Whearas for a neutron, with one up quark and two down quarks, the charge is:
$\frac{2}{3}-\frac{1}{3}-\frac{1}{3}=0$
Now, protons and neutrons combine to form the nucleus of an atom--that's basic enough. The combined number of protons and neutrons is the mass number--it essentially tells you how heavy the atom is. The reason elements such as lead are so heavy is that they have a relatively high mass number. Atomic mass, on the other hand, is a weighted average of the isotopes of an atom. It isn't a whole number--even though it is an average of whole numbers. The atomic number simply tells you how many protons are in the nucleus of an atom. For example, lead's atomic number is 82, so it has 82 protons in its nucleus.
That's the boring part of an atom. The real fun begins when you start to consider what happens around the nucleus--with these leptons (another type of particle) known as electrons. Electrons, in case you actually care, have a spin of 1/2 and a charge of -1 (big surprise there). However, what we really care about is where those electrons are and how they behave. Unfortunately for us, the best answer we can give is...we don't know. The issue is that we can't figure out where an electron is at any given moment. I read an analogy--I can't remember where--comparing this principle (the Heisenberg uncertainty principle) to an attempt to find a balloon floating around a dark room. The only way to find the balloon is to bump into it--thereby changing its position.
So what can we do to describe the behavior of electrons?
Well, the primary method used is to describe where the electrons are most likely to be at any given point in time. Mathematically, there is no issue with providing, say, a 90% certainty that an electron will be in a certain region at a certain time. Sure, there are equations that can provide these numbers--but I really don't feel like dealing with them in LaTeX right now. If you do, feel free to leave the string in the comments. I'll throw it in and give you credit.
Now, while an electron is flitting around in those regions where it either is or is not supposed to be, it does some pretty bizarre things. One of those involves acting like a wave, even though it's supposed to be a particle. (Even better--all particles act like a wave. That's called "wave-particle duality.") We don't observe larger objects acting like a wave because their mass is so comparatively huge that their wavelength is incredibly tiny. Why does the mass matter? Well, that's given by another equation:
$\lambda=\frac{h}{mv}$
This equation essentially assigns a wavelength to particles. It suggests that the wavelength of anything is equal to h (the Planck constant--the smallest possible unit of energy, a.k.a. "quanta") divided by the momentum that object has. Objects with a large amount of mass when compared to an electron have a large amount of momentum compared to the Planck constant, so their wavelength is comparatively tiny.
So, as we add on more and more electrons to the "electron cloud" (it sounds so high-tech), how do they join the group?
To understand this, you have to understand orbitals and energy levels. Let's look at a diagram of the periodic table, conveniently hot-linked to eat up your bandwidth:
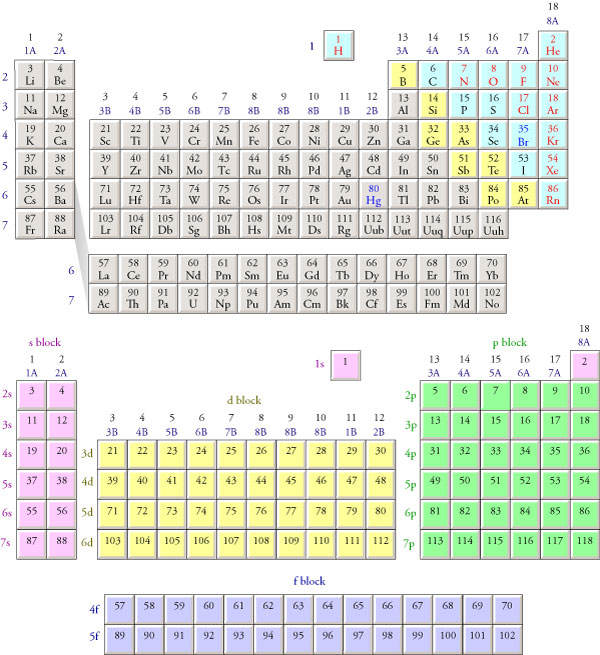
On the lower version, we notice these chunks called "blocks." Those each represent orbitals of energy levels. The purple writing along the left hand side shows, "1s, 2s, 3s..." and so on. This is the "s" orbital, which can hold two electrons--that's why each period has two purple cells. A hydrogen atom has one electron, in the 1s state (the s orbital of the first energy level). Helium will have two electrons, both in the 1s state. (This is commonly denoted as "1s2.") Continuing on to, say, Boron, we notice that it will have, once again, two electrons in 1s, two more in 2s, and another one in 2p. (Each orbital has a different shape, which is how they're distinguished, but I don't feel like going into that, do you?)
So then, the question becomes: How on earth was any of this ever figured out?
Well, once upon a time, the atomic model was a lot simpler. In fact, it was believed that the atom was the fundamental unit of all matter. A lot of this theory was developed and supported by a man named John Dalton. (I got into a little spat last year at Colorado State Knowledge Bowl over what exactly that theory was called, so it shall remain nameless here.)
As time went on, it became clearer that this was not the case. Another scientist named Rutherford discovered the electron by creating a stream of them through a vacuum tube between two plates. Because this stream was repelled by the negative end of a charged plate and attracted by the positive end, it was accepted that these subatomic particles were negatively charged.
Niels Bohr did some work with spectroscopy (analyzing the wavelengths of light emitted by elements) and designed another model. Our chemistry class actually repeated some of these experiments, and although I wasn't capable of taking beautiful pictures, I'll be more than happy to hotlink more in for you:
Bohr thought that electrons were emitting photons only at these specific frequencies because they were confined to certain orbits around the nucleus. Electrons could not be in the middle of two energy levels. When an electron was bumped up an energy level, it made a "quantum leap," causing it to emit a photon. (Incidentally, this kind of a spectrum, where photons are being emitted by the material being examined, is known as an emission spectrum.)
Here's the issue with the Bohr model, however: it's wrong. It doesn't explain the emission spectra of atoms with more than one electron (read: everything but hydrogen), and it gives electrons a definite position--which violates our old friend the Heisenberg Uncertainty Principle.
Well, I think that's all for now. If my abstract didn't make sense before, go back and read it again. It will now. I'm sure I'll be back soon with additions to make this post even longer!
To understand this, you have to understand orbitals and energy levels. Let's look at a diagram of the periodic table, conveniently hot-linked to eat up your bandwidth:

On the lower version, we notice these chunks called "blocks." Those each represent orbitals of energy levels. The purple writing along the left hand side shows, "1s, 2s, 3s..." and so on. This is the "s" orbital, which can hold two electrons--that's why each period has two purple cells. A hydrogen atom has one electron, in the 1s state (the s orbital of the first energy level). Helium will have two electrons, both in the 1s state. (This is commonly denoted as "1s2.") Continuing on to, say, Boron, we notice that it will have, once again, two electrons in 1s, two more in 2s, and another one in 2p. (Each orbital has a different shape, which is how they're distinguished, but I don't feel like going into that, do you?)
So then, the question becomes: How on earth was any of this ever figured out?
Well, once upon a time, the atomic model was a lot simpler. In fact, it was believed that the atom was the fundamental unit of all matter. A lot of this theory was developed and supported by a man named John Dalton. (I got into a little spat last year at Colorado State Knowledge Bowl over what exactly that theory was called, so it shall remain nameless here.)
As time went on, it became clearer that this was not the case. Another scientist named Rutherford discovered the electron by creating a stream of them through a vacuum tube between two plates. Because this stream was repelled by the negative end of a charged plate and attracted by the positive end, it was accepted that these subatomic particles were negatively charged.
Niels Bohr did some work with spectroscopy (analyzing the wavelengths of light emitted by elements) and designed another model. Our chemistry class actually repeated some of these experiments, and although I wasn't capable of taking beautiful pictures, I'll be more than happy to hotlink more in for you:
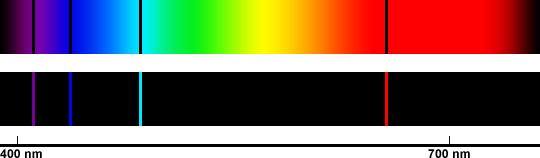 |
| Here's hydrogen. |
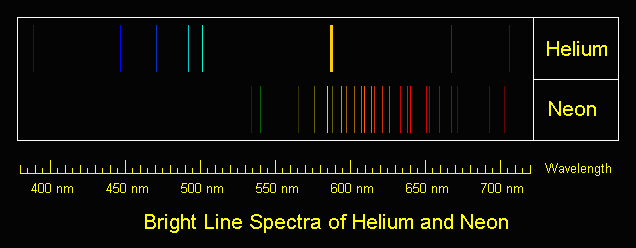 |
| Surprise! It's...helium and neon. |
Here's the issue with the Bohr model, however: it's wrong. It doesn't explain the emission spectra of atoms with more than one electron (read: everything but hydrogen), and it gives electrons a definite position--which violates our old friend the Heisenberg Uncertainty Principle.
Well, I think that's all for now. If my abstract didn't make sense before, go back and read it again. It will now. I'm sure I'll be back soon with additions to make this post even longer!
No comments:
Post a Comment