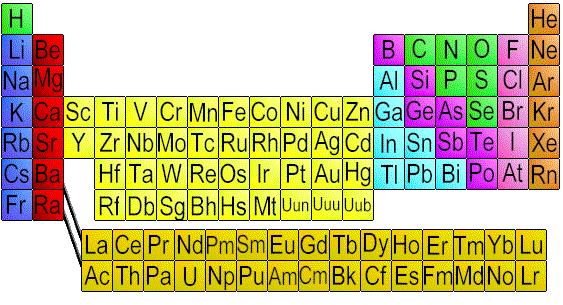
In every chemistry classroom (and I mean every chemistry classroom--I dare you to show me one without this) you'll find something called the periodic table (some editions include "of the elements"). It looks something like this:
At first glance, this seems to be a jumble of random letters. How can all of that have any meaning?
But on deeper investigation, we realize that it's actually quite powerful and organized. It's a tool that is used to group atoms with similar traits together. A basic overview: Going from left to right is a period, and up and down is a group. Across a period, the atomic number increases by increments of one. (Read: Going from left to right--across a period--one atom at a time, atoms gain one proton.) Atoms that are not transition metals (the yellow section in my image above, or groups 3-12--these elements have similar d-block configurations) are in groups with atoms with the same valence electron configuration (valence electrons are the electrons within an atom that are free to form bonds). By definition, this means that atoms in the same group have similar chemical properties, because valence electrons are what define chemical properties.
So, since groups have similar chemical properties...what are those chemical properties?
Well, I'm going to focus on the groups that have been named. Starting from the far left side, we'll find group 1--commonly known as "alkali metals." This group is part of the s-block of the periodic table, meaning that all of its outermost electrons are in the s orbital. Excluding hydrogen (and there is debate over whether or not hydrogen should even be a part of this group) the alkali metals are all extremely reactive metals.
Group 2, commonly known as the "alkaline earth metals," are also in the s-block, and also have all of their outermost electrons in the s orbital. They melt at extremely high temperatures, and are somewhat reactive.
There are other types of element as well, such as the transition metals, the metalloids, and non-metals. Transition metals tend to be mildly reactive, but conduct well because they bond in a way that involves sharing electrons, which in turn allows electrons to flow freely through them. The metalloids have some of the characteristics of metals--for example, both metals and metalloids tend to be solids, but metalloids tend to have a lower density than metals. Non-metals tend to be poor conductors, dull, and britle--each a characteristic unlike those of metals.
On the other end of the table, in group 17, we find the "halogens." As these elements tend to gain electrons, they are also extremely reactive. Once it reacts, however, the resulting molecule tends to be quite non-reactive because of the strength of its reaction. For example, Teflon is made by bonding fluorine with carbon.
And finally, of course, we have the "noble (inert) gases." These are named because of their tendency not to react with other elements. They are all odorless, colorless, non-reactive gases. Noble gases have a full outer shell, which is the reason for their innate inertness.
No comments:
Post a Comment